Latest Publications
EMC chaperone-CaV structure reveals an ion channel assembly intermediate. Chen Z, Mondal A, Aberemane-Ali, F, Jang S, Niranjan S, Montaño JL, Zaro BW, Minor, D.L., Jr. Nature 619 410-419 (2023)
Structural basis for CaVɑ 2δ:gabapentin binding. Chen Z, Mondal A, and Minor, D.L., Jr. Nature Struct. & Mol. Biol. 30 735-739 (2023)
Selective posttranslational inhibition of CaVβ1-associated voltage-dependent calcium channels with a functionalized nanobody. Morgenstern, T., Nirwan, N., Hernandez-Ochoa, E., Bibollet, H., Choudhury, P., Laloudakis, J., Ben-Johny, M., Bannister, R., Schneider, M., Minor, D.L., Jr., and Colecraft, H.M. Nature Communications 13:7556 (2022)
Definition of a saxitoxin (STX) binding code enables discovery and characterization of the Anuran saxiphilin family. Chen, Z., Zakrzewska, S., Hajare, H., Alvarez-Bullya, A., Abderemane-Ali, F., Bogan, M., Ramirez, D., O’Connell, L.A., Du Bois, J., and Minor, D.L., Jr. Proceedings of the National Academy of Sciences, USA 119:e2210114119 (2022)
Quaternary structure independent folding of voltage-gated ion channel pore domain subunits. Arrigoni, C., Lolicato, M., Shaya, D., Rohaim, A., Findeisen, F., Fong, L.-K., Colleran, C.M., Dominik, P., Kim, S.S., Schuermann, J., DeGrado, W.F., Grabe, M., Kossiakoff, A.A., and Minor, D.L., Jr. Nature Structural and Molecular Biology 29: 537-548 (2022)
Structural insights into the mechanisms and pharmacology of K2P potassium channels. Natale, A.M., Deal, P.E., and Minor, D.L. Jr. Journal of Molecular Biology 433:166995 (2021)
Differential effects of modified batrachotoxins on voltage-gated sodium channel fast and slow inactivation. MacKenzie, T.M.G., Abderemane-Ali, F., Garrison, C.E., Minor, D.L., Jr., and Du Bois, J. Cell Chemical Biology Dec 24;S2451-9456(21)00517-1 (2021)
Evidence that toxin resistance in poison birds and frogs is not rooted in sodium channel mutations and relies on ‘toxin sponge’ proteins. Abderemane-Ali, F., Rossen, N.D., Kobiela, M.E., Craig, R.A.II, Garrison, C.E., Chen, Z., O'Connell, L.A., Du Bois, J., Dumbacher, J.P., and Minor, D.L., Jr. J. Gen. Physiol 153:e202112872 (2021)
MINOR LAB RESEARCH
Our lab employs a range of biochemical, biophysical, structural, and chemical biology approaches to examine the molecular structures, functions, and biogenesis of various classes of ion channels including members of the voltage-gated potassium, voltage-gated calcium, voltage-gated sodium, and K2P channel families. Our research relies heavily on cryo-electron microscopy, X-ray crystallography, isothermal titration calorimetry, and other biophysical methods. Because ion channel structure is intimately tied to function, an equally crucial part of our efforts implements structure-based tests of ion channel function using electrophysiological recordings in live cells.
Because most channels suffer from poor pharmacological profiles that limit the ability to connect ion channel genes with their physiological functions, our lab also has a strong effort to develop novel ion channel modulators. The development of new selective inhibitors and activators of channel function should provide new tools for ion channel research and may lead to the development of novel, ion channel directed pharmaceuticals. Ion channels are the targets of many naturally occurring small molecule toxins such as saxitoxin (STX), the paralytic agent produced by oceanic ‘red tide’ blooms. The mechanisms by which animals that carry or regularly encounter such toxins resist poisoning remain poorly understood. In parallel with our interest in ion channels, we seek to uncover the molecular mechanisms of toxin resistance conferred by toxin binding proteins that act as ‘molecular sponges’ that neutralize toxins such as STX by sequestration. Understanding the basic rules for how proteins recognize STX and other toxins should aid in the development of new means to detect and counteract such toxins.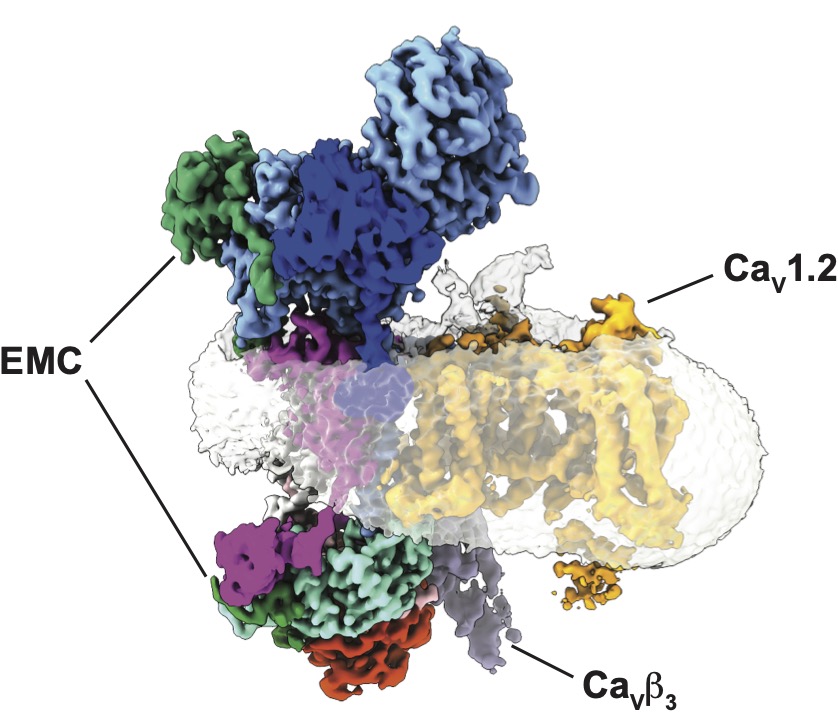
MINOR LAB LIFE
The Minor lab is located in the Smith Cardiovascular Research Building at the UCSF Mission Bay Campus in San Francisco. If you are interested in joining us, please see here.